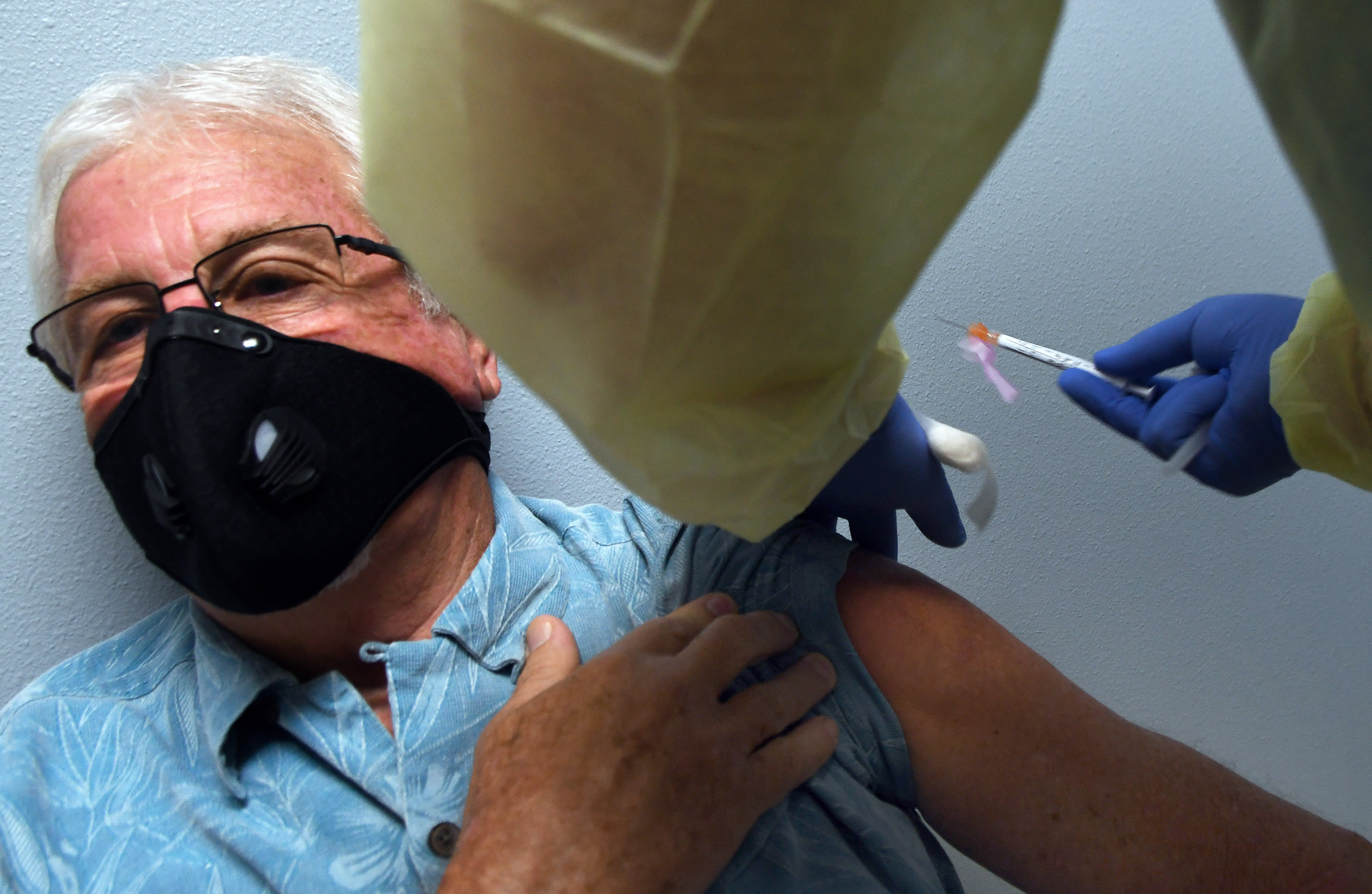
Tony Potts, a 69-year-old retiree living in Ormond Beach, receives his first injection as a participant in a Phase 3 COVID-19 vaccine clinical trial sponsored by Moderna at Accel Research Sites on August 4, 2020 in DeLand, Florida.
Paul Hennessy | NurPhoto | Getty Images
Moderna said on Friday it expects to produce 20 million doses of its experimental coronavirus vaccine by the end of the year.
The company continues to expect to make 500 million to 1 billion doses of the vaccine in 2021, Moderna said in a filing with the U.S. securities regulator.
There are currently no Covid-19 vaccines approved by U.S. regulators, although a handful are in late-stage trials to prove they are safe and effective.
Moderna’s Covid-19 vaccine is among the furthest in development and the company had enrolled 25,296 participants out of a planned 30,000 in its late-stage study as of Wednesday.
The Centers for Disease Control and Prevention anticipates that 35 million to 45 million doses of vaccines from the first two companies to receive authorization will be available in the United States by the end of this year.
Moderna has said it plans to seek emergency authorization for its vaccine’s use in high-risk groups if it proves to be at least 70% effective.




