Abbott Laboratories’ BinaxNow kit
Abbott Labs
Abbott Labs announced Tuesday that it is making its BinaxNOW Covid-19 rapid antigen test available to schools, universities, pharmacies and workplaces that require frequent and affordable testing. The company, that has been testing its U.S. employees weekly since October, is also sharing its testing blueprint with customers interested in learning the model it has used to get its workforce back to its facilities.
The company is in the final stages of completing its self-funded investment in U.S. manufacturing capacity to meet market demand. As the pandemic evolves, the need for rapid testing has only grown. To date at least 374,442 Americans have died from the coronavirus since the health crisis began and the nation is recording at least 244,300 new Covid-19 cases daily, based on a seven-day average calculated by CNBC using Johns Hopkins University data.
Rapid Covid testing could be a game-changer in the weeks and months ahead. Vaccine rollout in the U.S. has been slow. The fight to control the spread of the virus is expected to intensify as new infectious variants of the disease pop up in the U.S.
Since introduced in August, Abbott Labs has distributed 150 million BinaxNow Covid-19 antigen tests through the Department of Health and Human Services to states, territories, and targeted entities such as nursing homes, assisted living facilities, home health and hospice agencies, black colleges and universities and the Indian Health Service. Abbott will continue supplying HHS with a total of 30 million tests between now and March 2021.
In December, the Food and Drug Administration authorized Abbott Labs’ rapid Covid-19 test for at-home use, though doctors must prescribe the test for patients.
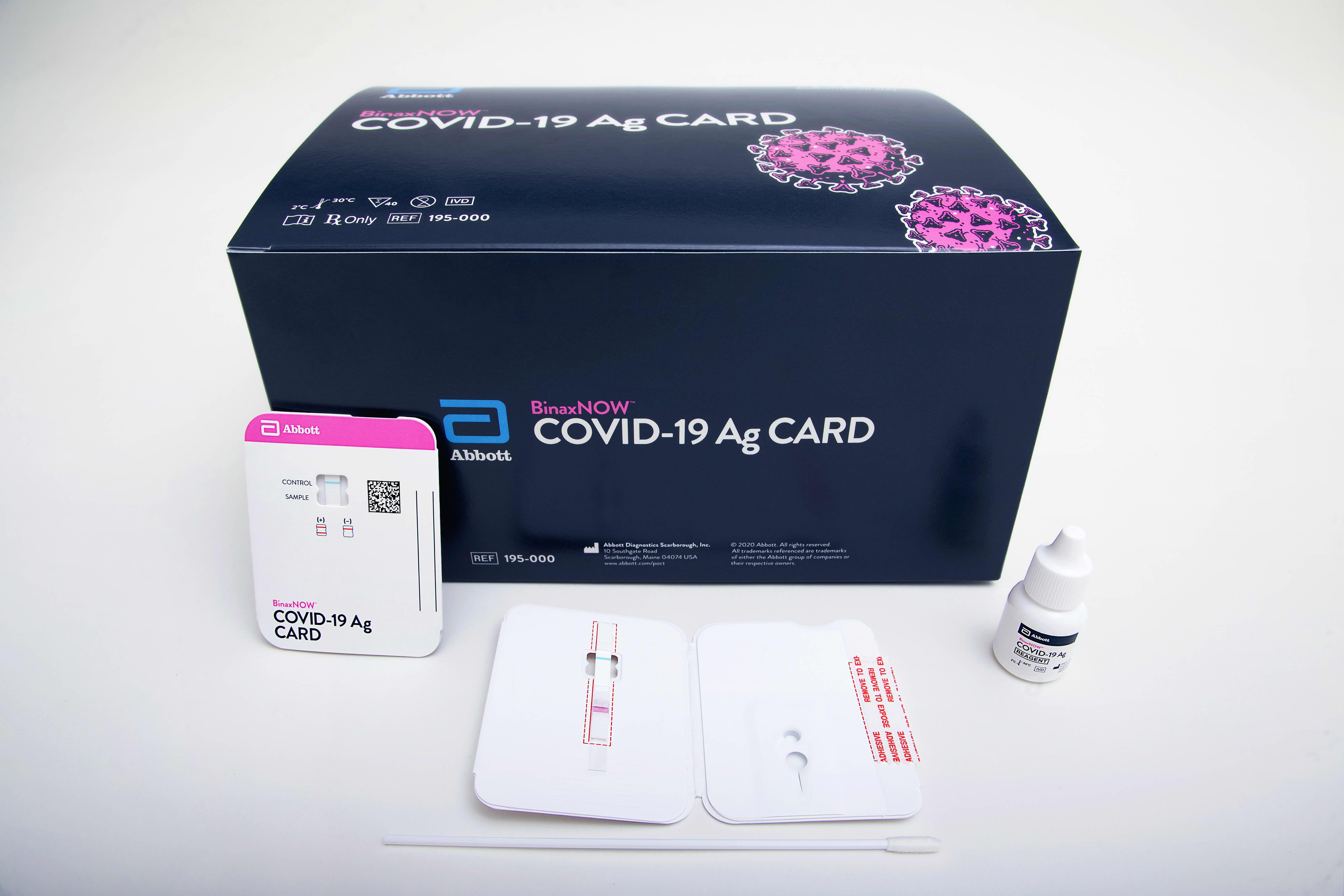
How it works
The size of a credit card and with no equipment required, Abbott’s $5 BinaxNOW Covid-19 test provides results in 15 minutes and detects the virus when people are most infectious and therefore at the greatest risk of spreading it to others. The BinaxNOW COVID-19 Ag Card is an assay for the qualitative detection of specific antigens to Covid-19 in the human nasal cavity. A simple nasal swab is used to collect specimens from people suspected of having an active infection.
The BinaxNOW test can be paired with the no-charge NAVICA app, which was developed by Abbott to allow people who test negative to display a temporary digital health certificate that is renewed each time a person is tested. People who test negative on BinaxNOW can receive a QR code (similar to a mobile boarding pass used to board an airplane) and organizations can scan and verify the information to manage entry into facilities that accept NAVICA.
The reliability of the BinaxNow test has been analyzed by the scientific community. A recent study from the University of California-San Francisco of BinaxNOW in a real-life setting showed performance of 93.3% sensitivity and 99.9% specificity at a Ct count of 30 and below. Ct counts are the number of times a PCR instrument must cycle through to amplify enough genetic material of the SARS CoV-2 virus for it to be detectable. The greater the amount of virus present (viral load), the fewer cycles required to detect the virus. A person with a higher viral load (and lower Ct count) is more likely to be infectious.
“Our intention with BinaxNOW and NAVICA has been to have greater impact on the country returning to some sense of normalcy with widespread availability in places where people need it most, such as schools and universities, workplaces, and pharmacies,” said Robert B. Ford, president and chief executive officer, Abbott. “While BinaxNOW has already benefitted many, it’s time for more people to see the benefits of fast and reliable rapid testing.”
The University of Wisconsin System will be the first customer in the U.S. to secure BinaxNOW at scale, procuring 480,000 tests over six months for use at its universities and branch campuses.
A safety net for the workforce
After having performed more than 300,000 tests on its employees nationwide, Abbott has gained important insights on managing workflow, reporting, and how to use NAVICA to allow people to store, access and display their test results, said Mary Moreland, executive vice president, human resources, Abbott. “This is information we’re willing to share as organizations want to learn more.”
The company has used the BinaxNOW test to boost health safety for its essential employees including scientists, engineers and manufacturing workers. “It has provided an extra level of protection and safety beyond CDC guidelines that include social distancing, wearing face masks and washing of hands,” Moreland added.
An Abbott Labs employee gets the BinaxNOW rapid Covid-19 antigen test at her workplace.
Abbott Labs
As she explained, employees must show a negative test result through the app on their mobile phones or have a scheduled test the following day before they can be admitted to the building.
Frank Weitekamper, vice president at Abbott’s transition organization who helped design the company’s testing rollout plan, noted that the company set up testing sites at 40 locations in the U.S., most often in conference rooms, and partnered with third-party occupational health professionals who conduct the tests. “You need a lot of resources for such a program and having a third-party partner assist in testing has proven to be a good strategy.”
So far, rapid testing of employees has not only boosted morale at Abbott Labs, it has also boosted productivity. “Because we do so much testing we are able to find a lot of asymptomatic employees who test positive for the virus,” Weitekamper said. “This allows us to quickly quarantine infected workers so they don’t infect others in the workplace.”
Mary Rogers, a virus hunter and principal scientist in Abbott’s diagnostics division, learned this firsthand. She took the test in October and was positive for Covid-19 although she exhibited no symptoms of the disease. “The early warning helped me ensure other members of my family and my coworkers never ended up getting the virus. I was able to immediately quarantine, test other members of my family for the disease, and take the necessary precautions to protect them so they wouldn’t get sick.”
Her tale reveals a shocking statistic. According to Moreland, 7 out of 10 workers who have tested positive for Covid after taking the BinaxNow test exhibited no symptoms of the disease. “This has been one of Abbott’s most surprising findings. Testing has enabled us to catch these so they could take precautions and not infect others in the workforce.”




