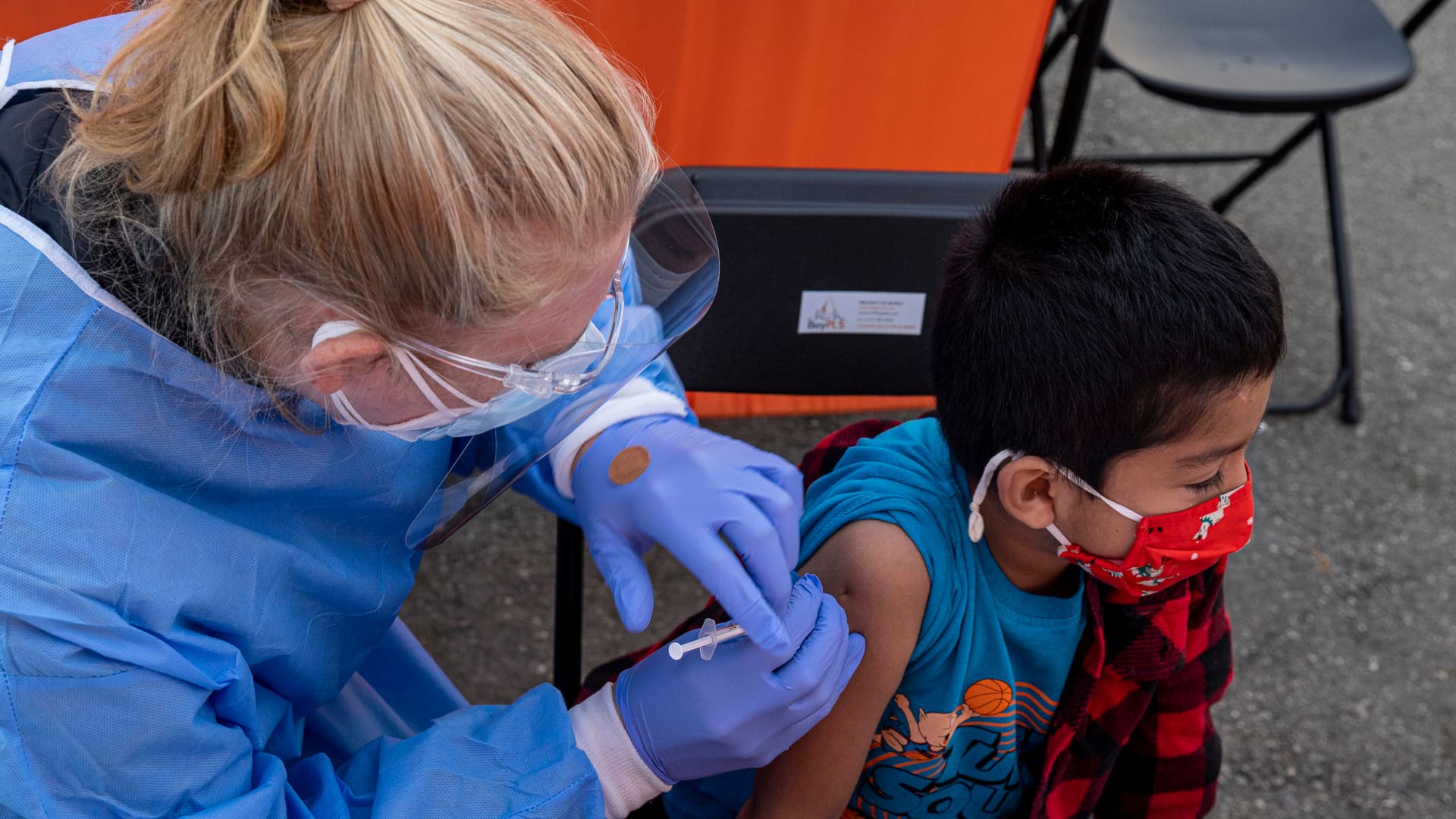
Pfizer and BioNTech‘s three-dose Covid vaccine for children six months to 5-years-old was 80% effective at preventing illness during the omicron wave, according to clinical trial results released Monday.
A third dose of the vaccine elicited a strong immune response and was well tolerated by the kids with a majority of the side effects mild to moderate, according to the companies.
BioNTech CEO Ugur Sahin said the companies plan this week to complete their application asking the Food and Drug Administration to authorize the vaccine. Pfizer CEO Albert Bourla said he hopes the vaccine will be available to younger kids as soon as possible.
The safety data is based on 1,678 children under age 5 who received a third shot at least two months after the second dose when omicron was the main variant in circulation. Pfizer examined a subset of children to determine the immune response of kids under age 5.
Kids under age 5 receive 3-microgram shots, one-tenth the dosage level for adults.
Pfizer and the Food and Drug Administration had originally sought to fast-track authorization of the first two doses in February so parents could start getting their kids vaccinated while clinical trial results from the third shot were still pending.
However, Pfizer delayed its application to wait on data from the third dose after the first two shots were only 30% to 40% effective, Bourla said in a podcast interview last month.
Children under age 5 are the only group in the U.S. that is not yet eligible for vaccination. Parents have been waiting months for the FDA to authorize the shots.
During the massive wave of omicron infection during the winter, children younger than age 5 were hospitalized with Covid at five times the rate of the pandemic peak, according to the Centers for Disease Control and Prevention. About 75% of kids under age 11 had been infected with Covid as of February, according to CDC data.
Moderna has also asked the FDA to authorize its two-dose vaccine for children under age 6. Its vaccine was about 51% effective against infection from omicron in children under age 2, and about 37% effective for kids ages 2 to under 6-years-old. However, Moderna said the antibody levels induced by the vaccine should translate into high levels of protection against severe illness.
The FDA’s committee of independent vaccine experts plans to meet in June to consider Pfizer and Moderna’s applications, with tentative dates set for June 8, 21 and 22. Bourla has said he hopes Pfizer’s vaccine for kids under 5 will receive authorization in June.




