Researchers demonstrate feasibility of using less cobalt, nickel in Li-ion batteries
The researchers explained that cobalt and nickel form a suitable crystal structure for LIBs’ key component—cathode materials. In these materials, lithium is easily and reversibly extracted/inserted.
The goal these days, however, is to find inexpensive elements that can be incorporated into the crystal structure.
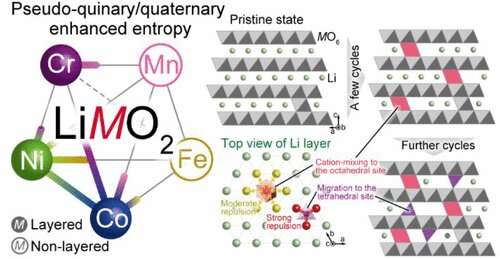
With this objective in mind, the Tohoku team decided to harness the energy gain from “configurational entropy”—a material’s state of randomness— and expanded the constituent elements’ solubility, synthesizing new composition electrode materials: LiCr1/4Mn1/4Co1/4Ni1/4O2 and LiCr1/5Mn1/5Fe1/5Co1/5Ni1/5O2. This significantly reduced the use of cobalt and nickel.
“Our approach unlocks the potential of other unused elements and will enable us to optimize multiple electrode properties simultaneously thanks to flexible material designs,” lead researcher Tetsu Ichitsubo said in a media statement.
Ichitsubo and his group also clarified the degradation mechanisms affecting the battery cycle with these new materials. In their view, this will serve as a guideline for developing novel high-performance components using the high-entropy strategy.
They say that whilst the cyclability and capacity did not match conventional LIBs at present, the ability to synthesize new electrode materials opens up further avenues for research into LIBs.




