Here’s how the ‘pause’ on J&J’s COVID-19 shot may affect the company’s earnings
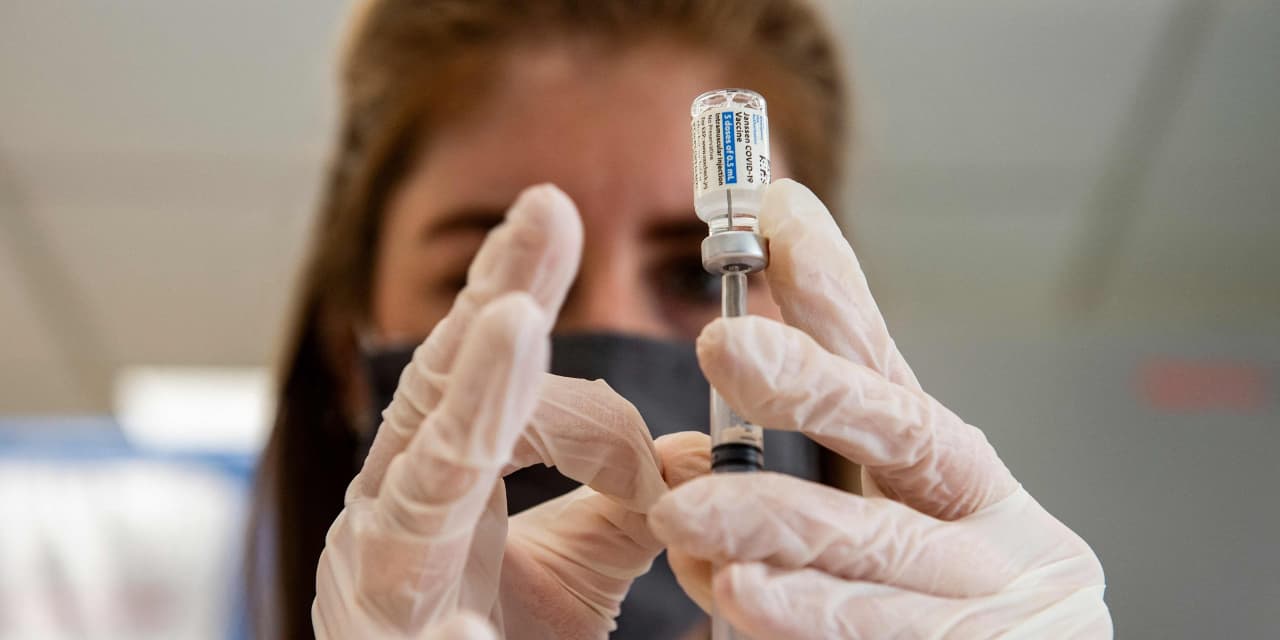
To what degree will Johnson & Johnson’s COVID-19 vaccine manufacturing issues and the federal “pause” over safety concerns weigh on investors’ mind when the company reports earnings next week?
The answer is probably not much, at least this time around.
Johnson & Johnson is expected to share its first-quarter earnings on Tuesday morning. This is the first time it has released earnings since the company’s single-dose coronavirus shot received emergency-use authorization in the U.S. in February. (That said, sales of the shot are largely already baked into analysts’ expectations because the vaccine is purchased in advance by governments.)
Shares of J&J JNJ gained 0.4% in trading on Friday.
Here is what investors will be paying attention to:
Whether J&J’s COVID-19 will be limited to use in a smaller group of people
In a surprise move, the Centers for Disease Control and Prevention and the Food and Drug Administration on Tuesday recommended a “pause” in administering the J&J vaccine until there was a better understanding of severe but ultra-rare blood clots reported in six women who have received this particular vaccine.
These six cases are out of the 6.8 million people in the U.S. who had received the shot, meaning the likelihood of an occurrence is roughly one out of every million shots, which has been described as “less than your chances of getting struck by lightning in a given year” by New York City health commissioner Dave Chokshi.
A CDC advisory committee voted this week to extend the pause as they gather more information about the cases, though analysts say they now expect the shot to be limited to use in adults over the age of 50 years old. The next emergency meeting of the CDC’s advisory committee to discuss what happens next with the J&J vaccine is scheduled for Friday, April 23.
The pause may have damaged trust — even temporarily — in the vaccine, with a recent poll conducted by the The Economist and YouGov finding that only 37% of respondents believe J&J’s vaccine is safe after the pause, compared with 52% prior to the pause.
The pause also had an immediate, though not long-lasting impact, on the stock.
“JNJ shares came under pressure in reaction to this news,” SVB Leerink said Tuesday. “However, there is no meaningful impact on earnings or downside risk to the guidance.”
The company’s stock closed on Tuesday at $158.48 on heavy volume — trading volume was more than three times higher that day than it had been the day before. This is a sharp fall from the share price closing at $165.01 on March 30, the day before the company confirmed a manufacturing mix-up of its vaccine at an Emergent Biosolutions Inc. EBS,
How J&J will address the shots’s manufacturing woes
On March 31, Johnson & Johnson confirmed that a batch of vaccines manufactured by Emergent, its U.S. manufacturing partner, did not meet quality standards, and its staff will now be supervising production at that facility.
By April 3, the company said it still expects to have delivered around 100 million doses of its vaccine to the U.S. government by June 1.
Biden administration officials have also sought to reassure the public that other vaccines are available at this time, including the mRNA vaccines developed by Pfizer Inc. PFE,
How the rest of the company’s medical portfolio fared in the first quarter
Because J&J’s business touches several different components of the health care system, its earnings performance is often viewed as a guide for other companies that report earnings at later dates.
J&J has three key business units: its medical devices business, which markets surgical products like hip and knee implants; its drugs business, which includes vaccines and its top-selling product, psoriasis drug Stelara, which generated $7.7 billion in sales in 2020; and its consumer business, which sells everything from baby shampoo to Neutrogena face wash.
The first quarter of medical sales in the U.S. tends to be less active because of the number of people who carry deductible plans that start over on Jan. 1 and are less eager to take on extra costs in the first part of the year. This particular quarter also faces a tough comparison to the first quarter of last year, in that COVID-19 was a growing concern but much of the state lockdowns hadn’t yet gone into effect.
“Demand is still likely to step down significantly from the relatively COVID-free results in Q1 2020 and from the seasonally strong Q4 results,” SVB Leerink’s Geoffrey Porges told investors in an April 16 note about the industry as a whole. “Demand for most specialty products has substantially recovered from COVID effects on starts, switches and re-treatment but in many cases will still fall short of pre-COVID levels.”
What to expect on Tuesday:
Earnings: Analysts tracked by FactSet expect that J&J earned $2.34 a share in the first quarter of 2021, up from $2.30 a year earlier.
Estimize, which crowdsources estimates from a range of parties, including buy and sell-side analysts, money managers, academics and more, is expecting EPS of $2.40
Revenue: The FactSet consensus predicts $22.0 billion in revenue for the first quarter of the year, up from $20.7 billion for the like quarter a year ago. This would include $200 million in sales for its COVID-19 vaccine for the quarter, for a total of $4.4 billion in total sales predicted for 2021.
Stelara is expected to generate about $2.2 billion in sales for the quarter, up from $1.8 billion in the first quarter of 2020, with total pharmaceutical sales hitting $12.1 billion in Q1 of 2021, compared to $11.1 billion in Q1 of 2020.
Estimize is forecasting revenue of $22.5 billion.
J&J’s stock is up 1.9% for the year, while the Dow Jones Industrial Average DJIA,




