Cassava Sinks After Lab Distances Itself on Alzheimer’s Data
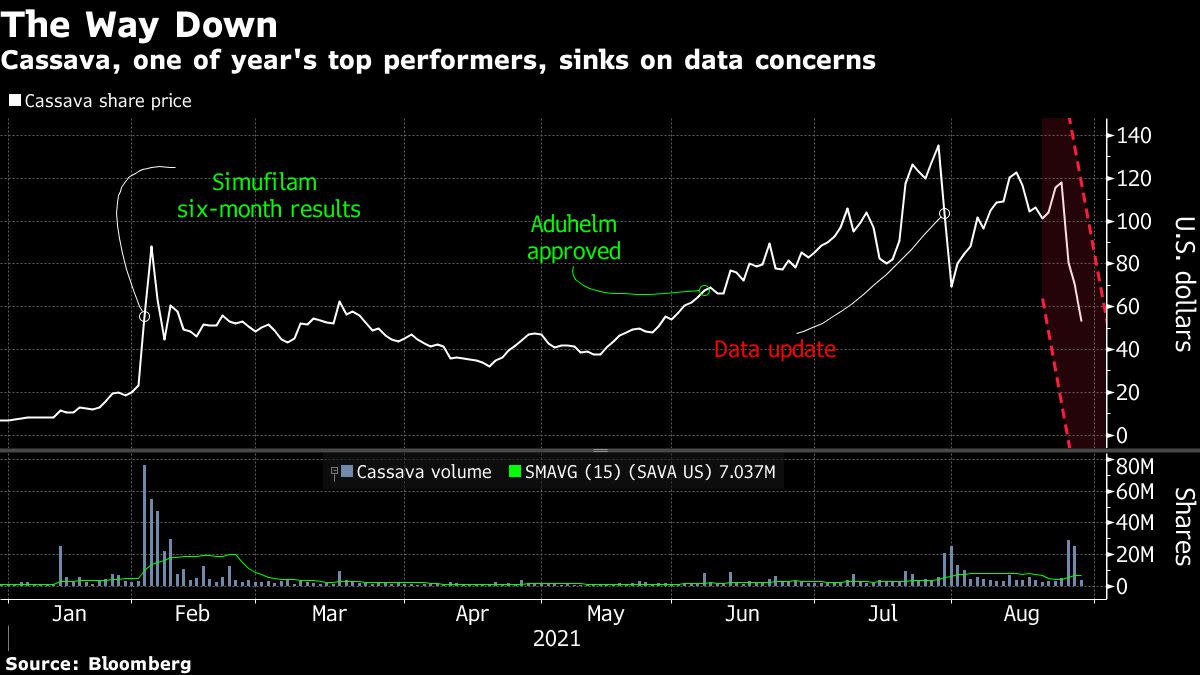
(Bloomberg) — Cassava Sciences Inc. plummeted as much 30% on Friday after a lab denied it had prepared recent results for the biotech’s lead product, an experimental Alzheimer’s disease treatment.
The denial counters a Wednesday statement from the company that Quanterix Corp. generated the results. Cassava shares have fallen about 50%, shaving off roughly $2.4 billion of value over the past three trading days. The fall comes after a former Securities and Exchange Commission lawyer petitioned the U.S. Food and Drug Administration to put a halt to Cassava’s trials of the drug on questions over the integrity of the results. The law firm behind the petition said their client holds a short position in Cassava.
Quanterix said it had performed some prior services to Cassava but “Quanterix or its employees did not interpret the test results or prepare the data charts presented by Cassava at the Alzheimer’s Association International Conference (AAIC) in July 2021 or otherwise,” according to a company statement.
Cassava said in a response Friday that Quanterix’s only duty in the trial was to perform sample testing. “We both had a job to do, we both did our respective jobs and we co-authored the results in an abstract at a major scientific conference,” Remi Barbier, Cassava’s chief executive, said in an email.
The stock is still one of the best performers this year, second only to AMC Entertainment Holdings Inc. among Russell 2000 Index members. The Austin, Texas-based company’s more than 700% rally was fueled by the approval of Biogen Inc.’s Aduhelm — the first new Alzheimer’s disease treatment in over a decade — as well as this year’s retail trading frenzy.
(Adds Cassava response, short position and updates shares.)
More stories like this are available on bloomberg.com
Subscribe now to stay ahead with the most trusted business news source.
©2021 Bloomberg L.P.




