In their view, this finding has implications for methane fuel recovery from some coal fields.
Based on the process described in this study, it is understood that coal forms when plant matter in wetland forests falls into the water and is quickly buried
In a paper published in the journal Science, the researchers explain that a methoxyl group consists of a carbon atom with three hydrogen atoms attached to an oxygen atom. The oxygen atom can attach to any number of places in a larger molecule. In the case of coal, it attaches to a carbon atom in one of coal’s ring arrangements.
Based on this process, it is understood that coal forms when plant matter in wetland forests falls into the water and is quickly buried. The organic material begins as peat, becomes lignite, then sub-bituminous, bituminous and finally anthracite as it is buried deeper and becomes more concentrated in carbon. Anthracite coal is mostly carbon, while lignite is still very vegetal.
According to Max Lloyd, lead author of the study, most coals used today in places like India and China are lignite or sub-bituminous because these are the only types easily and cheaply available, but these coals produce the largest amounts of greenhouse gases when burned.
As a solution to this problem, methane wells in these coal beds — coal bed methane (CBM) — is attractive as a bridge away from fossil fuels. The problem is that CBM production wells often have limited lifespans.
“The challenge to coal bed methane production is that creating wells is very expensive and the well may run dry in a month,” Lloyd said. “We don’t know why. Producers add more microbes or more nutrition (for the microbes), but that only works if those are the limiting factors, not if the coal itself is the limiting factor.”
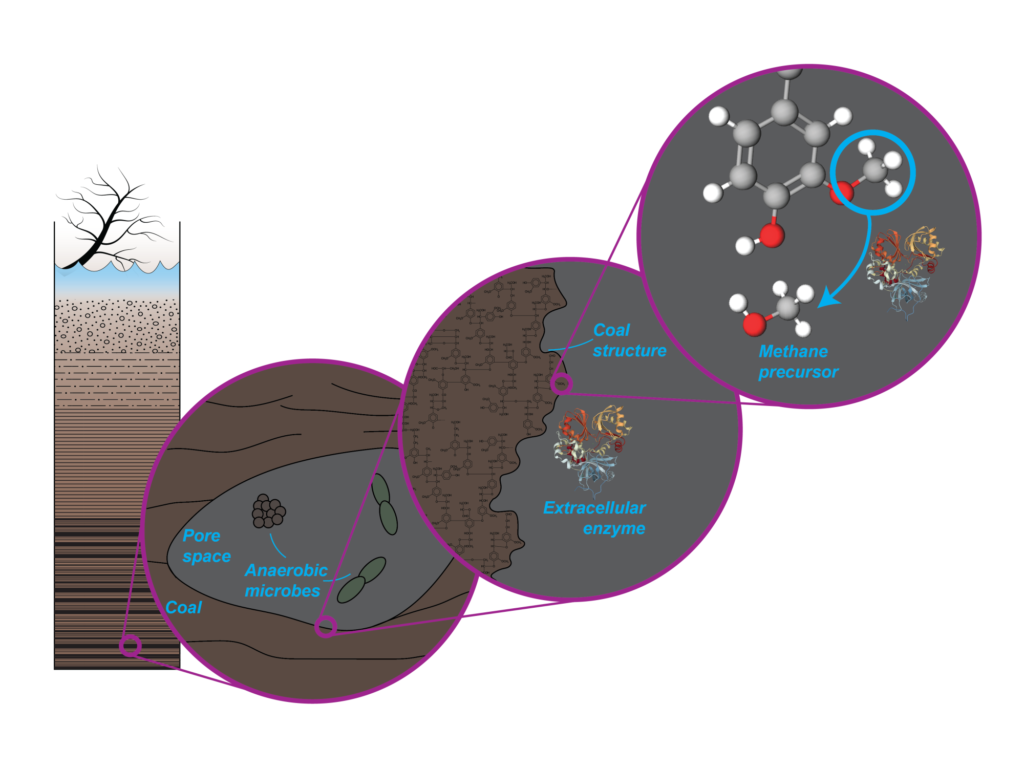
Lloyd was originally looking at the abundance of methoxyl groups in living or recently dead trees when he consulted his colleague Elizabeth Trembath-Reichert, who was working on microbes that consume methyl groups in coal. After they confirmed, using two methods, that the observations were real, Lloyd started looking for the same thing in coal from around the world.
Methoxyl groups in coal are turned into methane, but how methane forms from coal is poorly understood, according to the researchers. To better understand this process, they looked at stable isotopes of carbon in the methoxyl groups left behind.
Stable isotopes are the non-radioactive forms of an element that contain a varying number of neutrons in their nucleus. The isotopes of carbon that contain 12 and 13 neutrons are nearly identical, except that carbon 13, while less abundant in nature, is slightly heavier. Lloyd explained that biological organisms will generally prefer one isotope to the other so that what is left behind in the source will differ from the percentages of the isotopes normally found.
He and his colleagues, thus, looked at methoxyl groups in everything from wood to bituminous coal and noticed that the profile of isotopes did not match what would be found if the creation of methane had occurred due to heat, acidity or catalytic reactions, but they did match the patterns expected from microbial action.
“It turns out that aerobic microbes are great at degrading the rings in coal, but anaerobic microbes have no good way to take apart the rings,” Lloyd said. “So, one of the only things left for the anaerobes to do is cut off the methoxyl portions.”
These freed methoxyl groups are then converted to methane. But, once all the available methoxyl radicals are sheared from the rings, the microbes cannot get to anything else and the reaction stops and the well runs dry.
“The really interesting thing is that these microbes are releasing enzymes to cut off the methoxyl,” the scientist said. “They are degrading the structure extracellularly, which is limiting because coal is not a solution and the microbe can’t easily get everywhere in the coal structure.”
According to Lloyd and his colleagues, the depletion of methoxyl groups in coal over time points to the coal itself being the limiting factor in methane production. So, adding more microbes or nutrients will not produce more methane and another approach would be necessary.




