Water can also provide hydrogen “donors” in some varieties of ECR whereby the carbon atoms are combined with hydrogen to produce various species of hydrocarbons or alcohols.
Key to ECR is using the right catalyst or chemical substance whose structure and charge enable it to kick off or speed up a chemical reaction. Various different metals have been used as catalysts depending on which end product is desired. Catalysts employing just one type of metal include tin to produce formic acid, silver for carbon monoxide, and copper for methane, ethylene or ethanol.
However, the researchers say the performance of the process can be limited when ECR competes with the tendency of hydrogen atoms within the electrochemical splitting of water to pair up with themselves instead of joining up with the carbon atoms. This competition can lead to the production (or “selection”) of a different chemical end product than the one desired.
To avoid this issue, scientists have started looking into heterostructures that incorporate two distinct metals whose combined properties produce different or superior outcomes to either of the individual materials on their own.
Some of the heterostructures that have been tested so far for ECR include combining silver and palladium in a branchlike formation and various other combinations of two metals in sandwich-like, tube-like, pyramidal and other shapes.
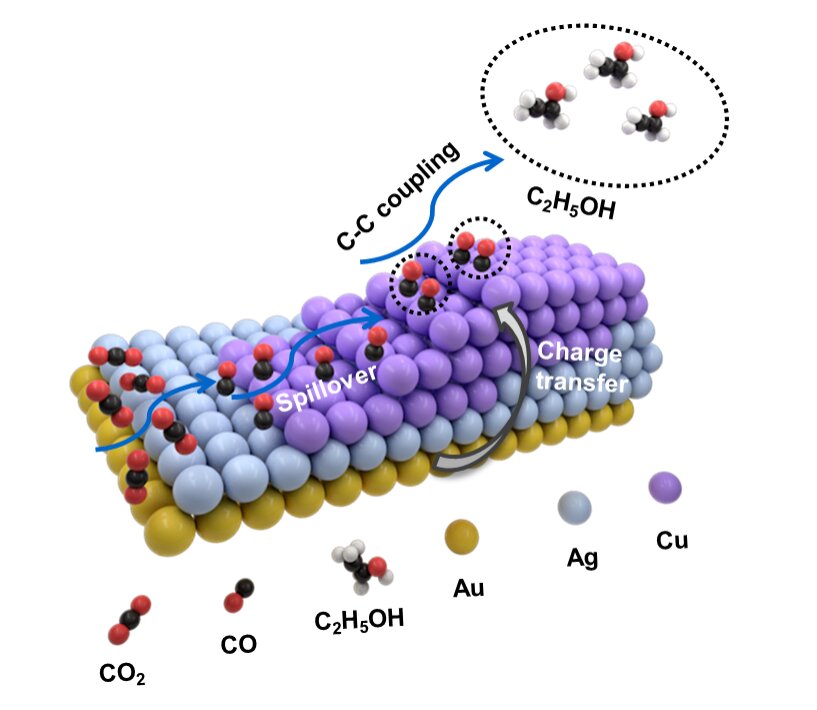
Previous research has been successful in employing bimetallic heterostructures that include copper—a metal that is very good at converting CO2 into products that use two carbon atoms. Among such bimetallic heterostructures, some incorporate silver-copper, zinc-copper and gold-copper with the latter enjoying particular selectivity success for methane, C2 and carbon monoxide.
“We thought if two metals were producing good results, then perhaps three metals would be even better,” Zhicheng Zhang, co-author of the study, said in a media statement.
The idea led Zhang and his colleagues to build a trimetallic nanostructure that combined gold, silver and copper and was asymmetric in form. The shape and precise ratio of the three metals can be altered via a growth method involving multiple steps. Specifically, gold “nanopyramids” were first synthesized and used as “seeds” for subsequent growth of various trimetallic structures involving different ratios of the three metals.
Following this arrangement, the researchers found that as a result of the unique form of their heterostructure design and by altering the ratios of the three metals, they could carefully tune the selectivity toward different C2-based products.
Production of ethanol, in particular, was maximized by using a heterostructure with the feeding ratio involving one atom each of gold and silver combined with five copper atoms.
In Zhang’s and his team’s view, this work sets out a promising strategy for the development of other trimetallic nanomaterials within ECR development.



