CDC advisors recommend Covid vaccines for children as young as 6 months
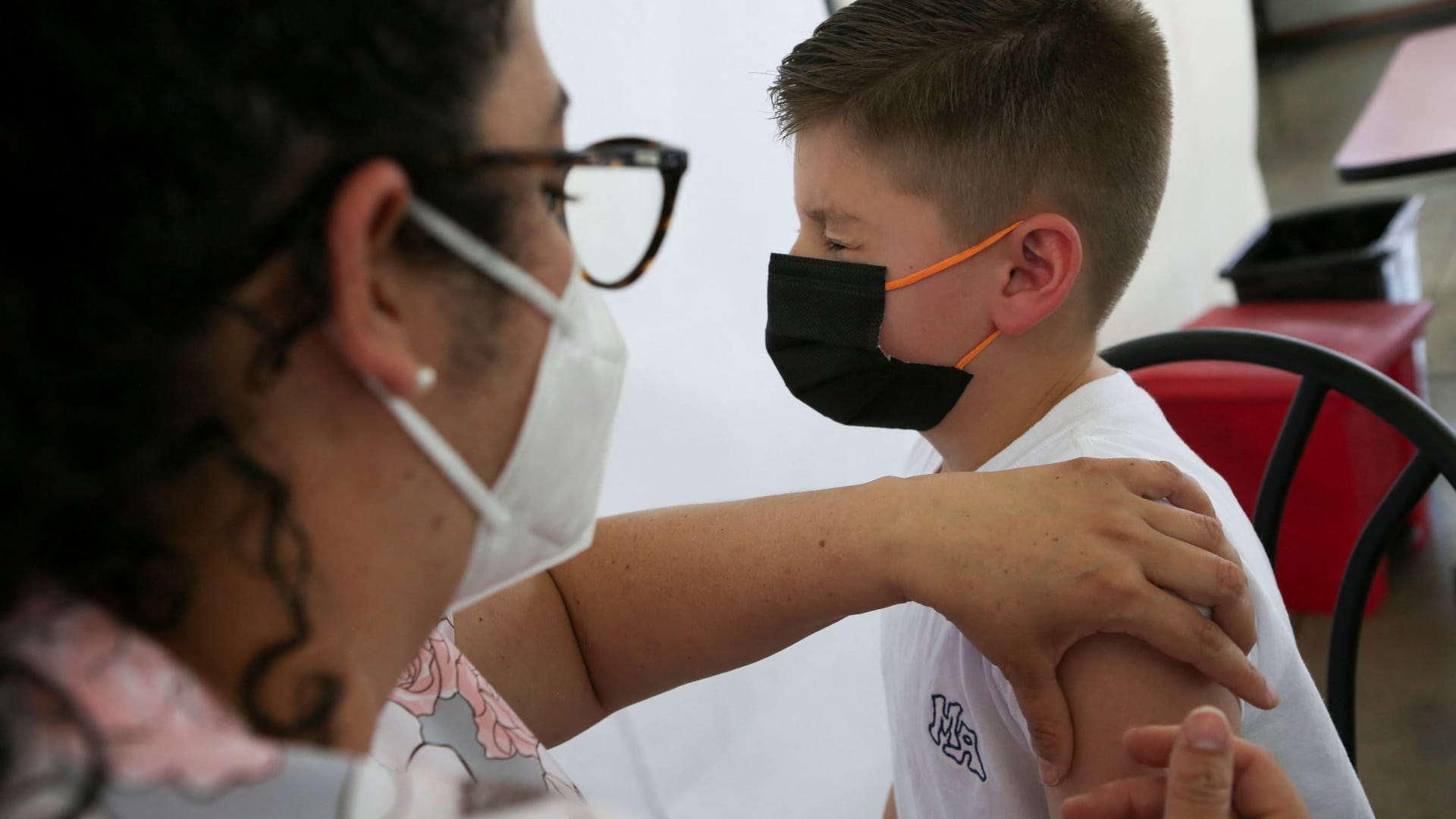
The Centers for Disease Control and Prevention’s independent vaccine experts on Saturday backed Pfizer’s and Moderna’s Covid-19 shots for children as young as 6 months.
The CDC committee voted unanimously to recommend the shots for use in infants through preschoolers after two days of meetings that were open to the public. CDC Director Dr. Rochelle Walensky is expected to sign off on the committee’s recommendation this weekend, which would allow pharmacies and doctor’s office to start administering the shots.
The White House expects vaccinations for children under age 5 to begin Tuesday, after the Juneteenth federal holiday. Appointment availability might be limited initially but every parent who wants to get their child vaccinated should be able to do so in the next few weeks, according to Dr. Ashish Jha, who oversees the Biden administration’s Covid response.
Nearly everyone in the U.S. is now eligible for Covid vaccination less than two years after the first shots were authorized for the elderly in December 2020.
“I am fully confident that vaccines should be recommended,” said Dr. Grace Lee, chairperson of the CDC committee. “We can clearly prevent hospitalizations and deaths. And I believe we have the potential to prevent long-term complications of infections that we don’t yet understand.”
The American Academy of Pediatrics, in a statement Saturday, strongly recommended that parents get their kids vaccinated and bring any questions or concerns they may have to their family doctor.
Covid risk for kids
Although Covid is normally less severe in children than adults, the virus can be life threatening for some kids. Covid is the fifth leading cause of death for children ages 1 to 4, according to CDC data. More than 200 children ages 6 months to 4-years-old have died from Covid since January 2020.
More than 2 million children in this age group have been infected with Covid during the pandemic, and more than 20,000 have been hospitalized, according to CDC data.
Hospitalizations of children under age 5 with Covid spiked during the winter omicron wave, hitting the highest level of the pandemic for this age group. The overwhelming majority of them, 86%, were admitted primarily due to the impact of Covid on their health, according to CDC data. In other words, they were not picked up in the data because they tested positive for the virus after admission for another health reason.
More than 50% of kids under age 5 who were hospitalized had no underlying medical conditions, according to CDC data. Nearly a quarter of kids hospitalized in this age group ended up in the intensive care unit.
Nearly 2,000 kids under age 5 developed multisystem inflammatory syndrome, or MIS-C, after Covid infection. MIS-C is a condition in which multiple organ systems – the heart, lungs, kidneys, brain, skin, eyes or digestive organs – become inflamed. Nine kids under age 5 have died from MIS-C.
“These very clear data just decimate the myth that this infection is not life threatening in this age group,” said Dr. Sarah Long, a committee member and pediatrician at St. Christopher’s Hospital for Children in Philadelphia.
Pfizer, Moderna vaccine differences
Pfizer’s vaccine is administered in three doses for children 6 months to 4 years old. The shots are dosed at 3 micrograms, one-tenth the level of what adults receive. Three shots were about 75% effective at preventing mild illness from omicron in 6-month- to 2-year-olds and 82% effective in 2- to 4-year-olds.
However, the data on the vaccine’s effectiveness is preliminary and imprecise because it is based on a small population of 10 kids, with estimates ranging from 14% to 96% protection against omicron. Dr. Bill Gruber, head of Pfizer’s vaccine research, said the antibody response observed in children post dose three, which was higher than people ages 16 to 25 who received two shots, should provide reassurance that the vaccine is effective.
“In the interest of sort of full transparency to parents, it’s to me appropriate to acknowledge the uncertainty around that,” committee member Dr. Matthew Daley said of the vaccine efficacy estimate.
It is crucial that parents who opt for Pfizer make sure their kids get the third shot to have protection against the virus. Two doses were only about 14% effective at preventing infection for kids under age 2, and 33% effective for those ages 2 to 4.
“I don’t want parents to get the impression that two doses is sort of good enough,” said Daley, a pediatrician who investigates vaccine safety.
Moderna’s vaccine is administered in two doses for children 6 months to 5 years old. The shots are dosed at 25 micrograms, one-fourth the level that adults receive.
Moderna’s vaccine was about 51% effective at preventing mild illness from omicron for kids 6 months to 2 years old, and about 37% effective for kids ages 2 to 5 years old. However, the company expects the vaccine to provide strong protection against severe illness because the kids had higher antibody levels than adults who received two doses.
Moderna is studying a booster dose that targets omicron for children in this age group with data expected on the shot’s safety and immune response expected in the fall, according to Dr. Rituparna Das, who leads Moderna’s Covid vaccine development.
The most common side effects from the vaccines were pain at the injection site, irritability and crying, loss of appetite and sleepiness, according to the FDA. Few children who received either shot developed a fever higher than 102 degrees Fahrenheit, and there were no cases of myocarditis, a type of heart inflammation, in Pfizer’s or Moderna’s trials.




