In a paper published in the journal Proceedings of the National Academy of Science, the researchers explain that their finding is important because hackmanite is considered a natural wonder material that is cheap and easy to synthesize and that has applications in personal UV monitoring and X-ray imaging.
“In this research, we found out for the first time that there is actually a structural change involved in the colour change process as well,” Mika Lastusaari, co-author of the study, said in a media statement. “When the colour changes, sodium atoms in the structure move relatively far away from their usual places and then return back. This can be called ‘structural breathing’ and it does not destroy the structure even if it is repeated a large number of times.”
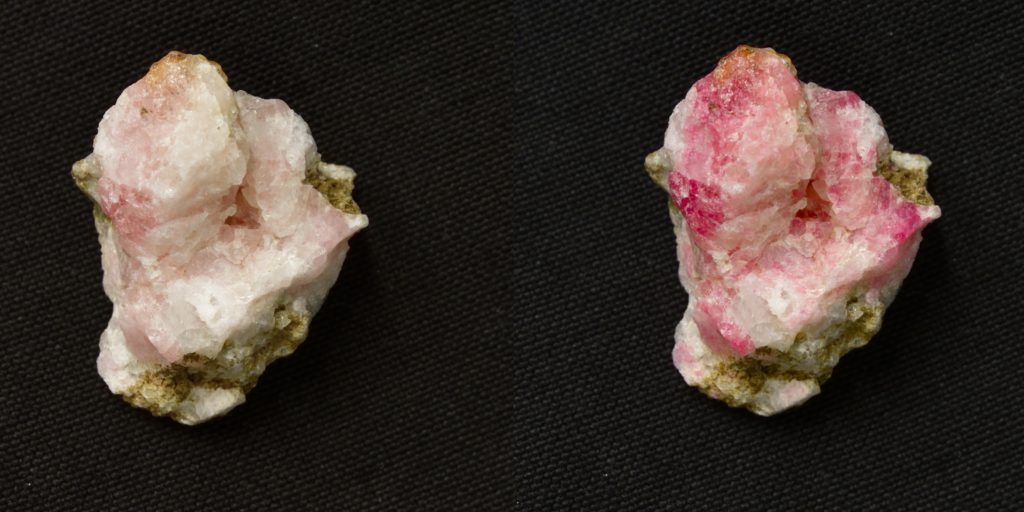
According to Lastusaari, the durability is due to the strong three-dimensional cage-like overall structure of these minerals, which is similar to that found in zeolites. In detergents, for example, the cage-like structure enables zeolite to remove magnesium and calcium from water by binding them tightly inside the pores of the cage.
“In these colour-changing minerals, all processes associated with the colour change occur inside the pores of the zeolitic cage where the sodium and chlorine atoms reside. That is, the cage-like structure allows atomic movement inside the cage while keeping the cage itself intact,” co-author Sami Vuori said. “This is why minerals can change colour and revert back to their original colour practically indefinitely.”
Vuori pointed out that previously, it has been known that scapolite changes colour much faster than hackmanite, whereas tugtupite’s changes are much slower. Based on the results of their work, the Finnish scientists understood that the speed of the colour change correlates with the distance that the sodium atoms move.
In their view, these observations are important for future material development because now they know what is required from the host structure to allow the control and tailoring of the colour change properties.
“There were no characterization methods available for the research on colour-changing minerals, which is why we have developed new methods by ourselves. However, it is difficult to interpret the results unambiguously based on experimental data alone,” Lastusaari said. “In fact, we could not have reached the present conclusions without strong support from theoretical calculations, since only the combination of experimental and computational data shows the whole picture.”
Thanks to these findings, Lastusaari and his group are currently exploring numerous applications for hackmanite, such as possibly replacing LEDs and other light bulbs with the natural mineral and using it in X-ray imaging.
One of the most interesting avenues that the researchers are currently looking into is a hackmanite-based dosimeter and passive detectors for the International Space Station, intended to be used to measure the radiation dose uptake of materials during space flights.
“The strength of hackmanite’s colour depends on how much UV radiation it is exposed to, which means that the material can be used, for example, to determine the UV index of Sun’s radiation,” Vuori explained. “The hackmanite that will be tested on the space station will be used in a similar fashion, but this property can also be used in everyday applications. We have for example already developed a mobile phone application for measuring UV radiation that can be used by anyone.”




